Urethral Balloon Catheters Market Growth Outlook 2026–2036 Driven by Standardized Urology Care
Global urethral balloon catheters demand rises steadily as hospitals standardize urology workflows and prioritize patient safety through 2036.
NEWARK, DE, UNITED STATES, January 17, 2026 /EINPresswire.com/ -- Market Overview: Steady Growth Anchored in Clinical Protocols
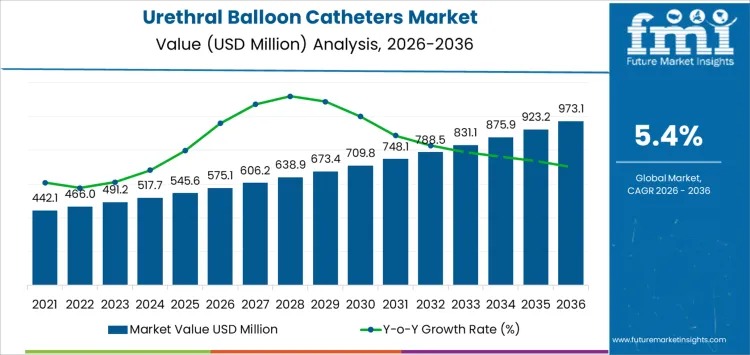
The global Urethral Balloon Catheters Market is projected to grow steadily as hospitals and long-term care facilities expand standardized urology and post-operative care protocols. Market value is estimated at USD 575.1 million in 2026 and is expected to reach USD 973.1 million by 2036, advancing at a 5.4% CAGR. Growth is closely tied to hospital procedure volumes and clinical workflow adoption rather than promotional activity or rapid product redesigns.
Unlike many medical device segments driven by frequent innovation cycles, urethral balloon catheters follow long replacement and approval timelines. Once a catheter type is validated within hospital protocols, procurement teams typically maintain the same specifications for years to reduce retraining requirements and procedural variability. This creates predictable, recurring demand aligned with patient throughput.
Request For Sample Report | Customize Report | Purchase Full Report –
https://www.futuremarketinsights.com/reports/sample/rep-gb-31370
Key Market Drivers Supporting Long-Term Expansion
Hospital purchasing behavior defines demand patterns in the urethral balloon catheters market. Adoption is driven by operational consistency and patient safety priorities rather than brand-led differentiation.
Primary growth drivers include:
- Rising urology and post-operative procedure volumes in hospitals and outpatient centers
- Standardization of catheter protocols to reduce clinical errors
- Growing aging population requiring bladder management and long-term catheterization
- Increased emphasis on infection control, traceability, and sterility assurance
Once embedded into clinical pathways, catheter usage becomes routine, reinforcing stable reorder cycles across hospital networks.
Procurement Criteria: Reliability Over Promotion
Hospitals evaluate urethral balloon catheters using functional and operational benchmarks. Nurses and clinicians assess insertion comfort, balloon inflation accuracy, leak resistance, and packaging integrity. Procurement teams focus on shelf life, lot traceability, and supplier service availability.
Manufacturers therefore compete on:
- Balloon material strength and biocompatibility
- Lumen precision and drainage reliability
- Consistent lot-to-lot quality and regulatory compliance
- Training support and dependable supply schedules
Aggressive pricing strategies play a secondary role, as hospitals prioritize reducing complication rates and workflow disruptions over marginal cost savings.
Product and Application Trends
- Two-way catheters account for the largest share of demand due to their widespread use in routine urology and post-operative bladder drainage. Their adoption supports standardized care protocols and reduces procedural variability.
- Three-way catheters serve irrigation-intensive applications and require additional staff training, limiting their use to specific clinical scenarios.
- Specialty catheters address niche needs but involve higher regulatory scrutiny and closer supplier engagement.
From an application standpoint:
- Urology procedures represent the leading share of demand due to high procedural frequency
- Post-operative care generates recurring consumable demand through prolonged indwelling use
- Other specialized applications create targeted opportunities for premium or customized solutions
Regional Growth Outlook
- Growth is strongest in Asia Pacific, supported by expanding hospital infrastructure and rising patient volumes. India and China lead regional expansion as healthcare systems invest in urology departments and standardized care pathways.
- North America shows steady growth driven by replacement cycles and preference for advanced, compliant catheter systems.
- Europe reflects mature, guideline-driven demand, with Germany demonstrating stable replacement-led growth.
These regional patterns highlight how infrastructure expansion and clinical standardization directly influence adoption rates.
Competitive Landscape: Embedded Relationships Matter
Key players such as BD, Teleflex, Coloplast, Cook Medical, and B. Braun compete during early evaluation and protocol-definition stages. Once approved, catheter selection becomes deeply integrated into hospital workflows, making supplier changes costly and infrequent.
Competitive positioning depends on:
- Documentation quality and regulatory support
- Training programs and technical assistance
- Distribution reach and inventory reliability
Long-term success is achieved through inclusion in hospital formularies and network-wide procurement agreements rather than isolated department sales.
Long-Term Market Perspective
The urethral balloon catheters market demonstrates how clinically essential devices generate consistent growth without rapid innovation cycles. Expansion toward nearly USD 1 billion by 2036 reflects cumulative adoption across hospitals, surgical centers, and long-term care facilities. Demand remains closely linked to patient care volumes, standardized protocols, and operational efficiency, ensuring resilience and predictability for manufacturers and distributors alike.
Related Reports:
Endoscopic Probe Disinfection Market- https://www.futuremarketinsights.com/reports/endoscopic-probe-disinfection-market
Veterinary Hospital Market- https://www.futuremarketinsights.com/reports/veterinary-hospital-market
Veterinary Eye Care Market- https://www.futuremarketinsights.com/reports/veterinary-eye-care-market
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware - 19713, USA
T: +1-347-918-3531
Why FMI: https://www.futuremarketinsights.com/why-fmi
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.